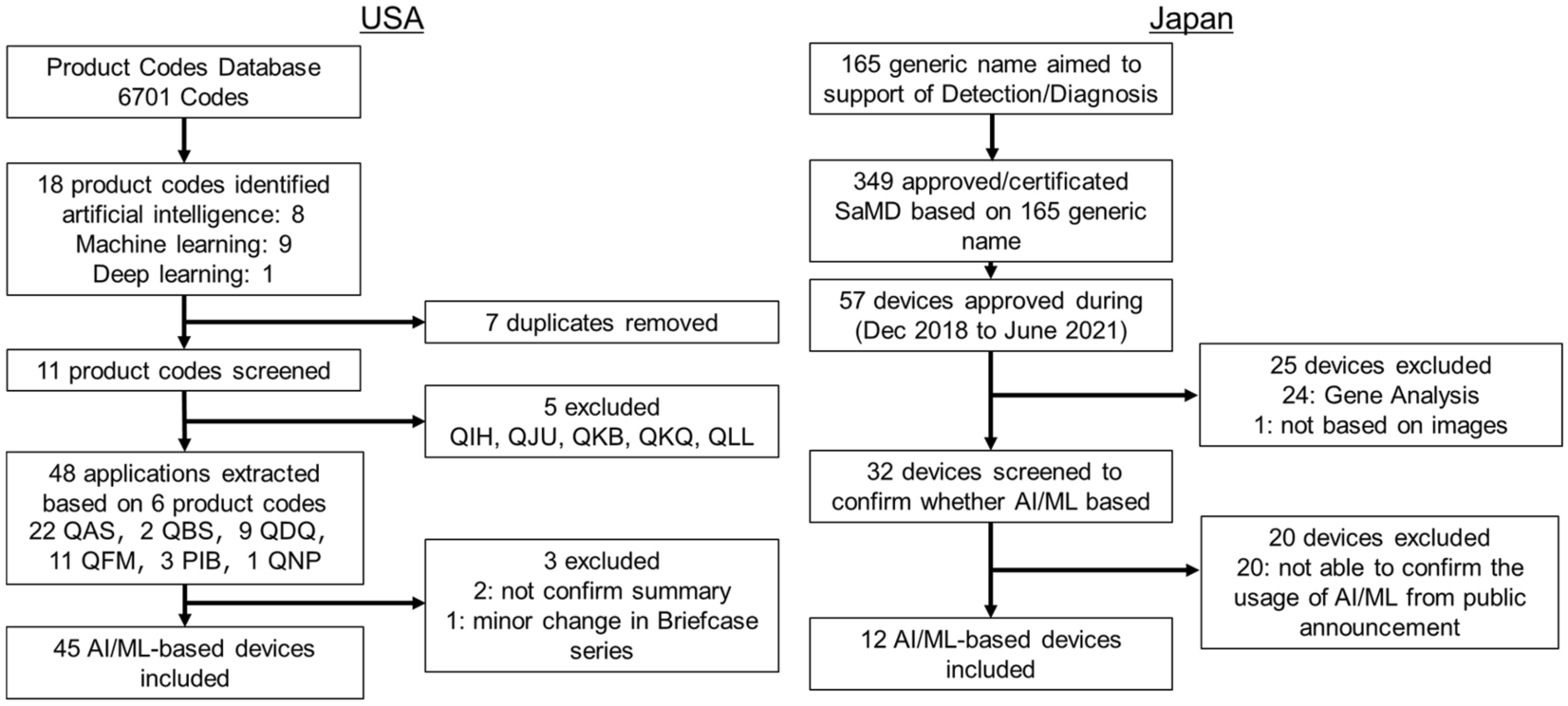
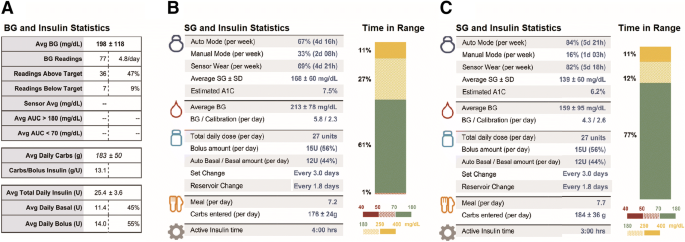
August 31, 2020 Medtronic Minimed, Inc. Nicole Birch Senior Regulatory Affairs Specialist 18000 Devonshire Street Northridge, CA

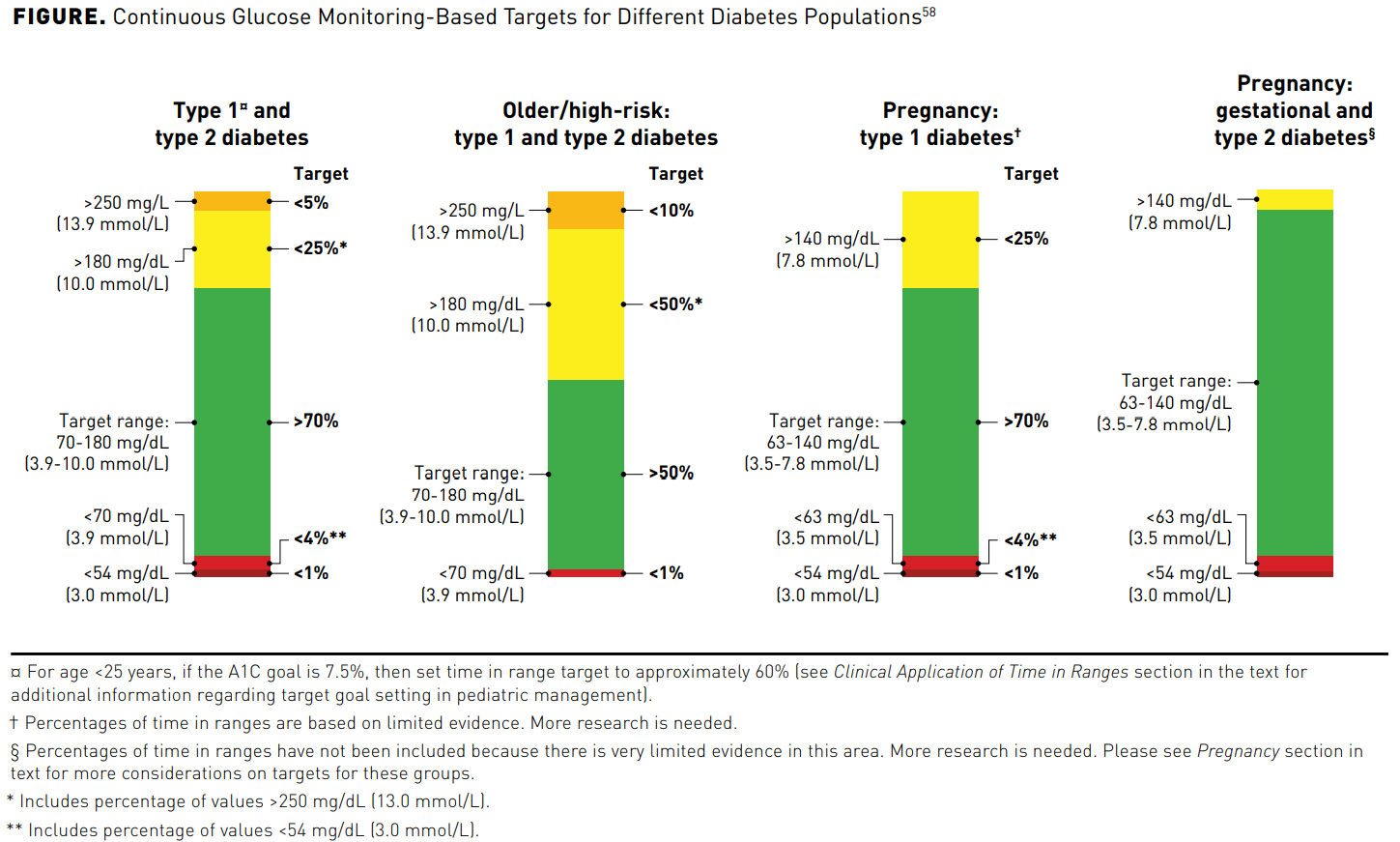
Continuous Glucose Monitors and Automated Insulin Dosing Systems in the Hospital Consensus Guideline
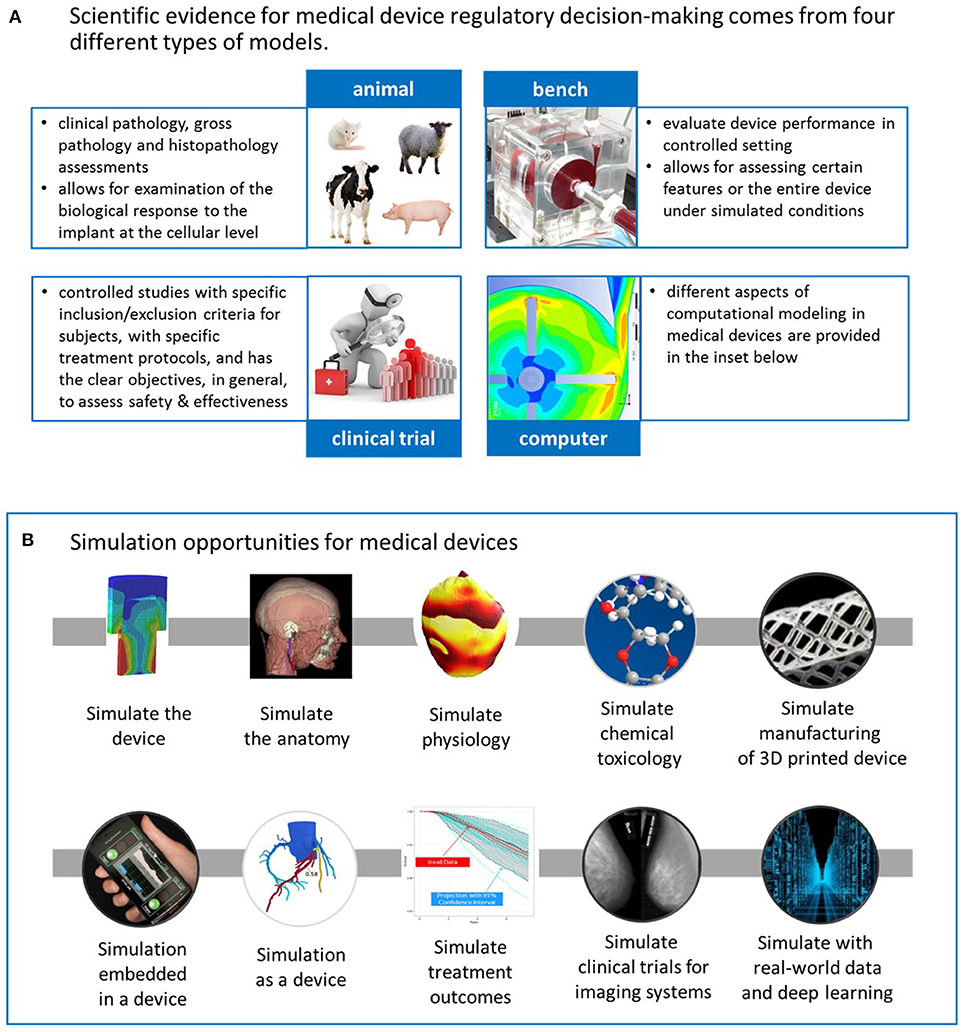
Frontiers | Advancing Regulatory Science With Computational Modeling for Medical Devices at the FDA's Office of Science and Engineering Laboratories

A Hybrid Closed-Loop Insulin Delivery System for the Treatment of Type 1 Diabetes - CADTH Issues in Emerging Health Technologies - NCBI Bookshelf
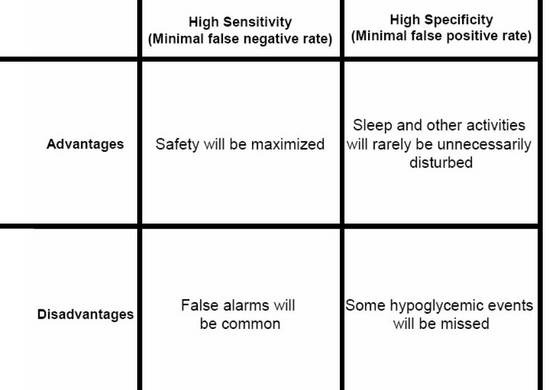
Monitoring Technologies- Continuous Glucose Monitoring, Mobile Technology, Biomarkers of Glycemic Control - Endotext - NCBI Bookshelf

Long-Term Clinical Effectiveness of a Drug-Coated Balloon for the Treatment of Femoropopliteal Lesions | Circulation: Cardiovascular Interventions